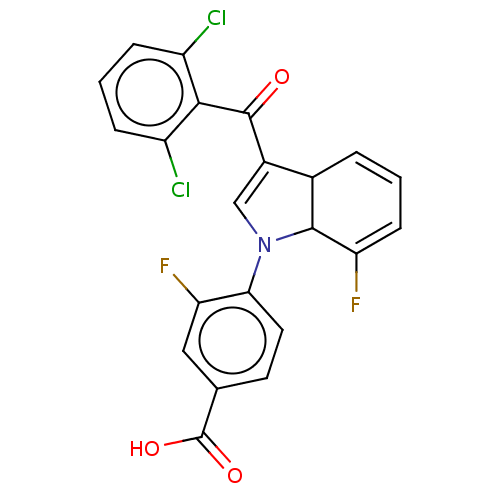
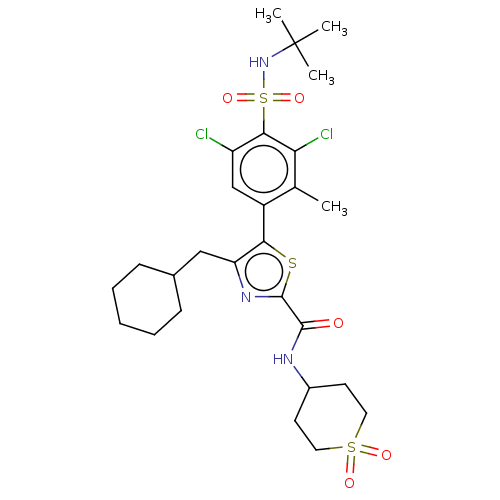
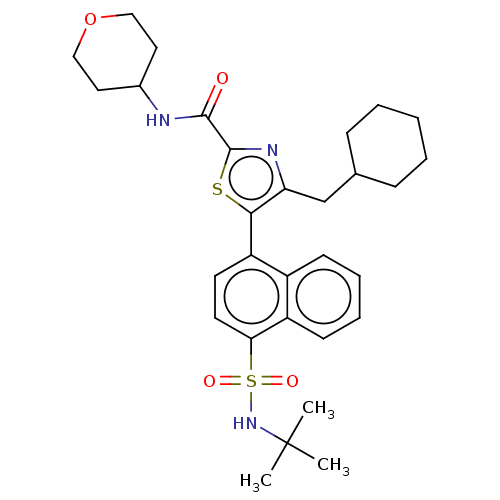
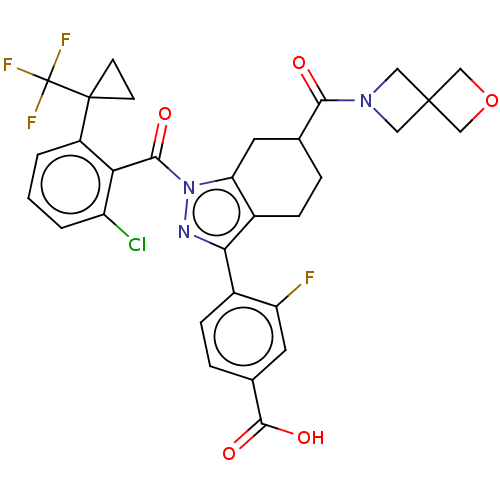
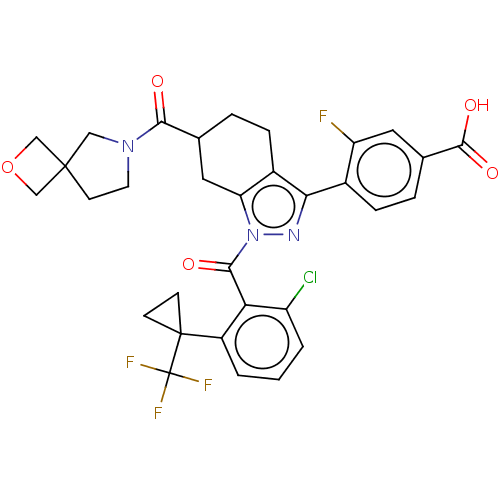
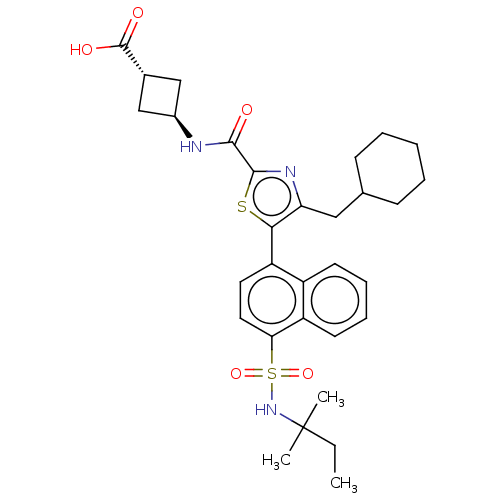
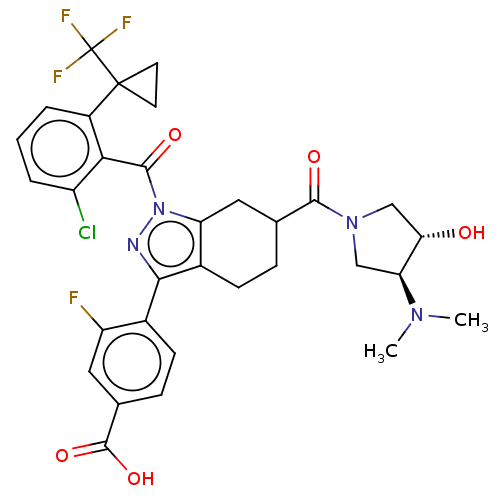
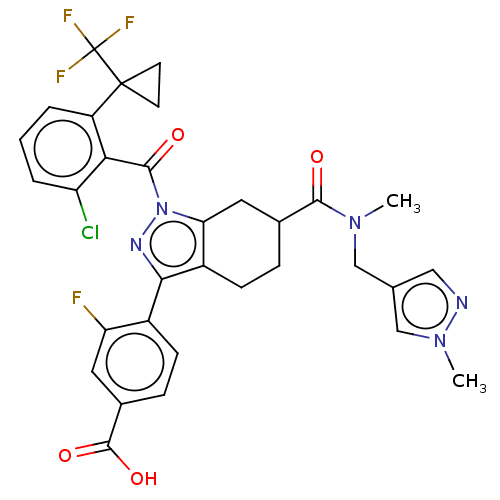
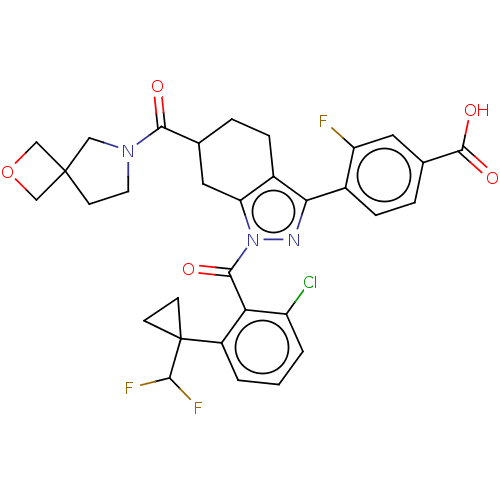
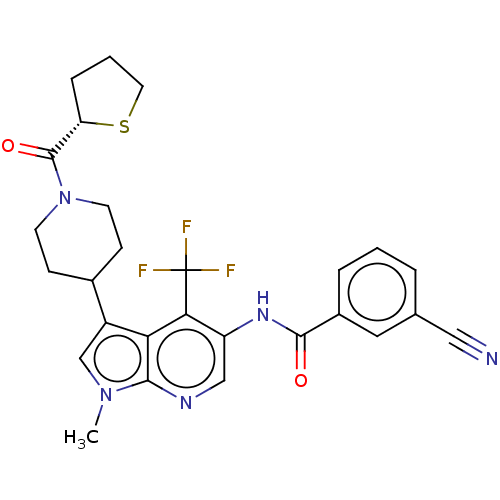
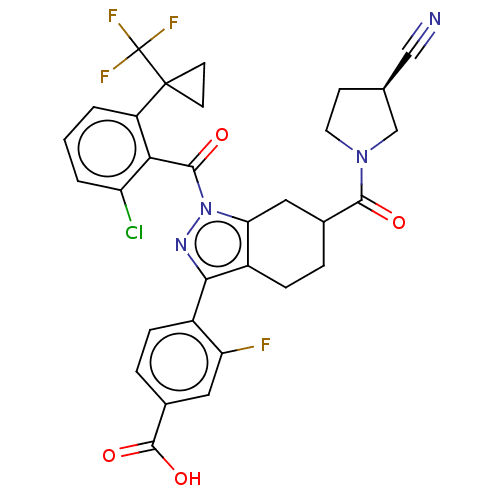
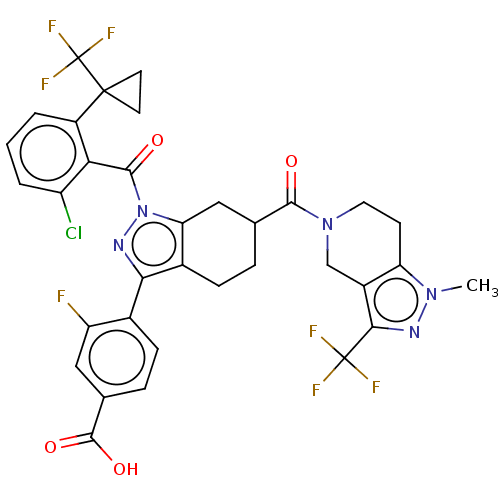
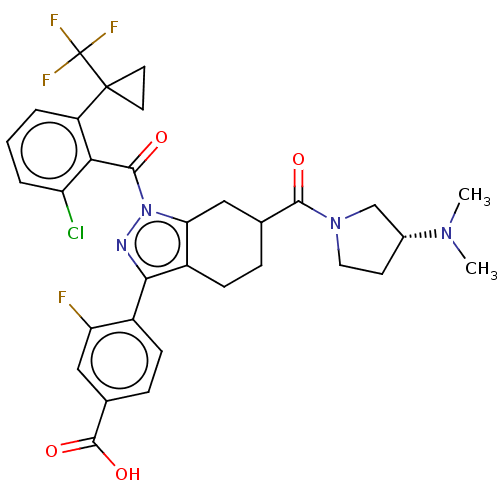
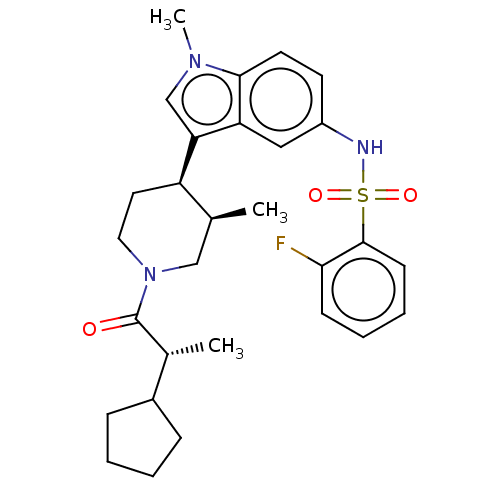
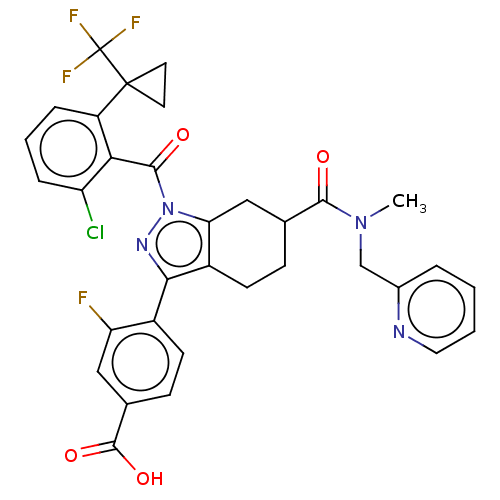
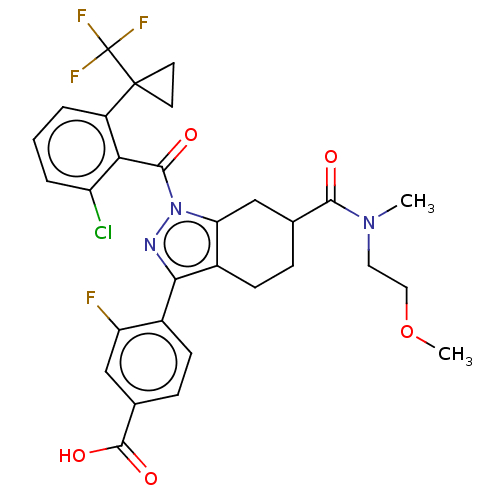
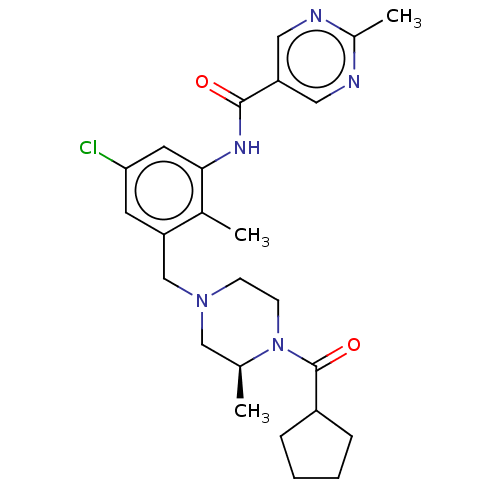
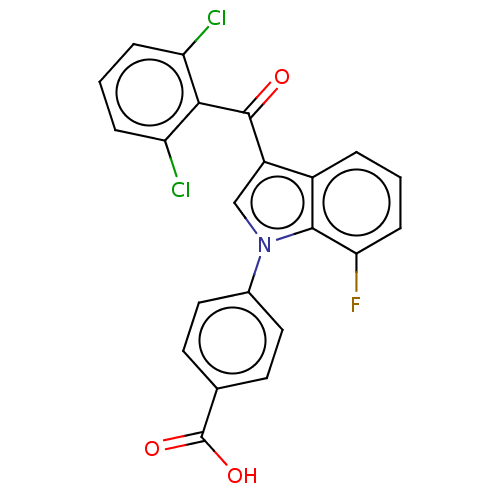
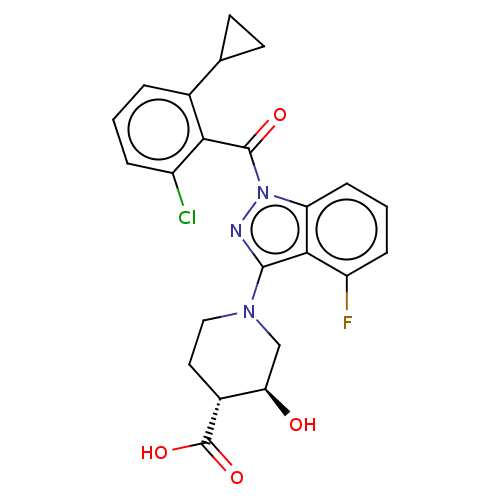
Affinity DataIC50: 0.100nMAssay Description:Binding affinity to recombinant human RORgammat transfected in human HEK293T cells incubated for 24 hrs by dual-glo luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inverse agonist activity at human His6-tagged RORgammat LBD (264 to 518 residues) assessed as reduction in biotinylated RIP140 co-activator recruitme...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.615nMAssay Description:The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding...More data for this Ligand-Target Pair
TargetIsoform 2 of Nuclear receptor ROR-gamma (RORgT)(Homo sapiens (Human))
Phenex Pharmaceuticals
US Patent
Phenex Pharmaceuticals
US Patent
Affinity DataIC50: 0.631nMAssay Description:Cells were incubated for additional 16 h before firefly (FF) luciferase activities were measured sequentially in the same cell extract using a Dual-L...More data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMAssay Description:Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMpH: 7.5 T: 2°CAssay Description:Specifically, in one embodiment the aforementioned assay was performed as outlined below. The assay was carried out in black polystyrene, 384-well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:The activity of compound of the invention can be determined by a co-activator recruitment by TR-FRET (time-resolved fluorescence resonance energy tra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In general, the assay is based on the interaction between N-terminally Six-Histidine-tagged-RORC2 ligand binding domain (6-His-RORC2 LBD), expressed ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:Inverse agonist activity at human GAL4 DBD-fused RORgammat LBD (237 to 497 residues) expressed in HEK293T cells assessed as reduction in ROR mediated...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inverse agonist activity at recombinant 6His-tagged human RORgamma LBD (264 to 518 residues) assessed as inhibition of biotinylated RIP140 co-activat...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inverse agonist activity at GST-tagged RORgammat LBD (unknown origin) using fluorescein-D22 co-activator peptide as substrate incubated for 1 hr by T...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Allosteric inhibition of recombinant His6-tagged RORgammat LBD (unknown origin) expressed in Escherichia coli BL21(DE3) assessed as inhibition of bio...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair

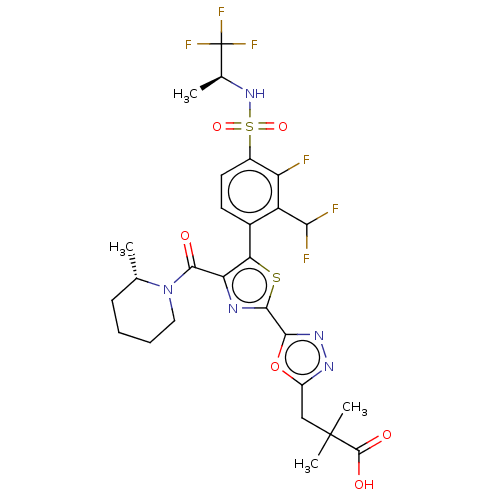



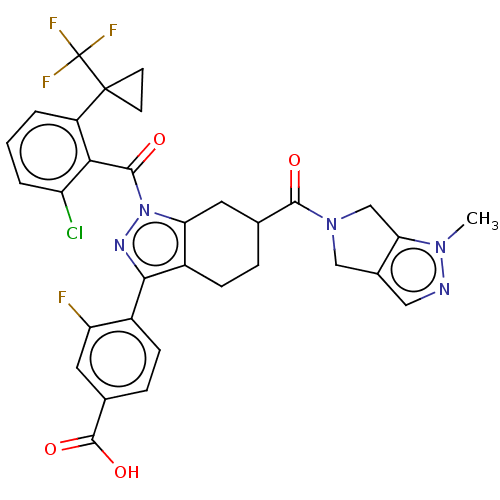
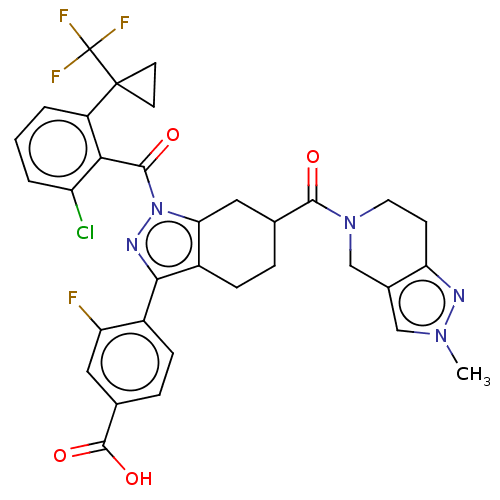

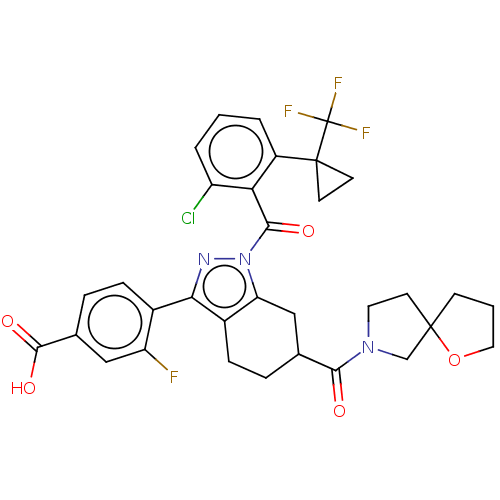
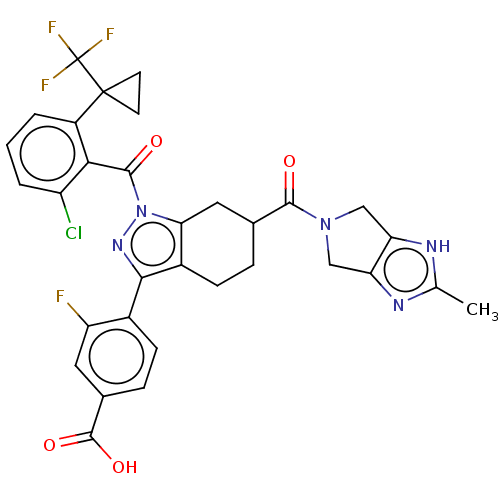
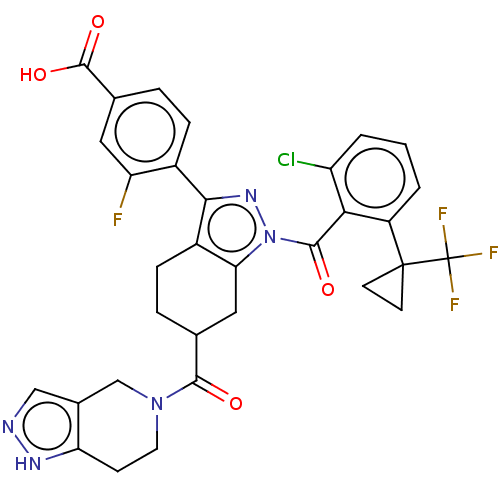
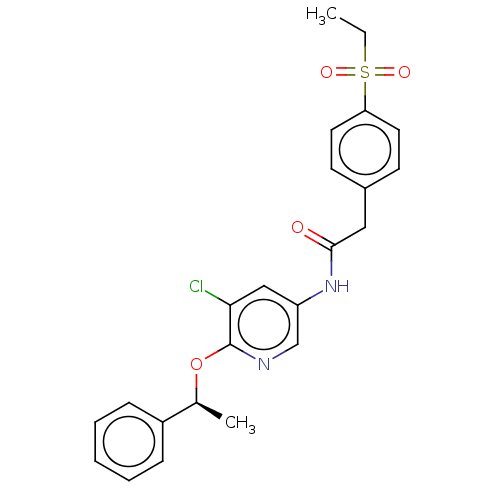
 3D Structure (crystal)
3D Structure (crystal)